So far cultured meat has been burgers – the next big challenge is animal-free steaks

It’s relatively easy to grow a bunch of animal cells to turn into a burger. But to grow a steak made of cultured meat is a trickier task. Bioengineers must create organized, three-dimensional tissues.
The meat you eat, if you’re a carnivore, comes from animal muscles. But animals are composed of a lot more than just muscle. They have organs and bones that most Americans do not consume. They require food, water, space and social connections. They produce waste.
Farmers spend a lot of energy and resources to grow complex organisms, creating waste in the process, only to focus on the profitable cuts of meat they can harvest.
It would be easier, more humane, less wasteful, to produce just the parts people want. And with cell biology and tissue engineering, it is possible to grow just muscle and fat tissue. It’s called cultured meat. Scientists provide cells with the same inputs they need to grow, just outside an animal: nutrients, oxygen, moisture and molecular signals from their cell neighbors.
So far researchers have cultivated bunches of cells that can be turned into processed meat like a burger or a sausage. This cultured meat technology is still in the early phases of research and development, as prototypes are scaled-up and fine-tuned to prepare for the challenges of commercialization. But already bioengineers are taking on the next tougher challenge: growing structured cuts of meat like a steak or a chicken cutlet.
What meat’s made of
If you look at a piece of raw meat under the microscope, you can see what you’re eating on the cellular level. Each bite is a matrix of muscle and fat cells, interlaced with blood vessels and enrobed by connective tissue.
The muscle cells are full of proteins and nutrients and the fat cells are full of, well, fats. These two cell types contribute to most of the taste and mouth-feel a carnivore experiences when biting into a burger or steak.
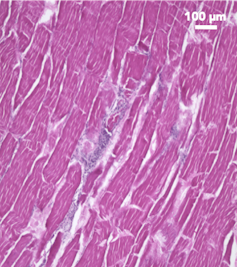 Section of turkey stained to show cellular-level organization skeletal muscle tissue – also known as meat.
Section of turkey stained to show cellular-level organization skeletal muscle tissue – also known as meat.Natalie Rubio
The blood vessels supply an animal’s tissue with nutrients and oxygen while it’s alive; after slaughter, the blood adds a unique, metallic, umami nuance to the meat.
The connective tissue, composed of proteins like collagen and elastin, organizes the muscle fibers into aligned bundles, oriented in the direction of contraction. This connective tissue changes during cooking and adds texture – and gristle – to meat.
The challenge for cellular agriculture researchers is to emulate this complexity of meat from the bottom up. We can grow muscle and fat cells in a petri dish – but blood vessels and connective tissue don’t spontaneously generate as they do in an animal. How can we engineer biomaterials and bioreactors to provide nutrient diffusion and induce organization so we end up with a thick, structured cut of meat?
Cultured-meat burgers are the first step
To create any cultured meat, researchers take small – think marble-sized – amounts of tissue from a cow, pig or chicken and isolate individual cells. Then, bioengineers like me put the cells in plastic flasks and give them nutrients, oxygen and moisture while housing them at body temperature. The cells are happy and can divide exponentially, creating more and more cells.
When grown on plastic, the cells will continue to divide until they exist on all of the available surface area. This results in a crowded layer that’s one cell thick. Once the cells stop dividing, they start to mature. Muscle cells fuse together to create long muscle fibers and fat cells begin to produce lipids. Researchers can combine a bunch of these cells together to create processed meat products, like burgers, hot dogs and sausages.
Animal cells alone can replicate most of the meat experience. But without blood vessels and connective tissue, you don’t end up with an organized, three-dimensional tissue – and that’s what you need for structured cuts of meat, like steak, chicken breast and bacon.
To overcome this challenge, scientists can use biomaterials to replicate the structure and function of blood vessels (for nutrient and oxygen transfer) and connective tissue (for organization and texture). This area of research is called scaffold development.
 Providing some structure for cells to grow on will get cultured meat from hamburger to steaks.
Providing some structure for cells to grow on will get cultured meat from hamburger to steaks.Tyler Olson/Shutterstock.com
Scaffolds are the secret ingredient for steaks
The concept of scaffolds originates in the field of tissue engineering for medical applications. Scientists combine cells and scaffolds to produce functional biomaterials for research, toxicology screening or implants.
These biomaterials can take different forms – films, gels, sponges – depending on what properties are desired in the resulting tissue. For example, you could grow skin cells on a flat collagen film to create a skin graft to help burn victims or bone cells in a hydroxyapatite sponge for bone regeneration.
For medical applications, scaffolds generally need to be safe for implantation, must not induce a response from the body’s immune system, be degradable and capable of supporting cell growth.
For food applications, the design considerations of scaffolds are different. They should still support cell growth, but it’s also important that they are inexpensive, edible and environmentally friendly to produce. Some common biomaterials for food applications include cellulose from plants, a carbohydrate called chitosan from mushrooms and a carbohydrate called alginate from algae.
Here’s one “recipe” for cultured meat that I’ve worked on in the lab. First, create an appropriate scaffold. Isolate chitosan from mushrooms and dissolve it in water to create a viscous gel. Put the gel in a tube and expose one end to a cold substance, like dry ice or liquid nitrogen. The whole tube of gel will slowly freeze, starting at the cold end. The frozen gel can then be freeze-dried by a vacuum pulling on the gel at very low temperatures, ultimately creating a dry sponge-like material. The directional freezing process creates a sponge with small, long, aligned pores resembling a bundle of straws – and also muscle tissue.
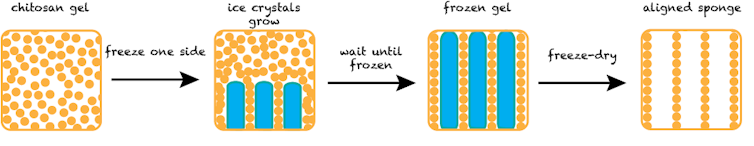 The simplified process for creating a chitosan sponge with aligned pores.
The simplified process for creating a chitosan sponge with aligned pores.Natalie Rubio, CC BY-ND
Then, instead of growing meat on flat plastic, you can transfer the cells to this three-dimensional sponge to provide more surface area for growing thicker tissue. The pores can also help distribute nutrients and oxygen throughout the tissue. So far with this technique, my lab has been able to produce small bits of meat less than a centimeter square – a little small for a cookout but a strong start.
Other scaffold possibilities include growing cells within alginate-based fibers, gels or sponges. Or technicians can rinse plant cells off of plants in a process called decellularization and repopulate the cellulose framework that’s left behind with animal cells.
Once researchers find materials and methods that work really well, we’ll work on creating larger batches. At that point, it’ll be a game of scaling up the process and bringing down the cost so cultured meat products can be cost-competitive with farmed meat products.
It’s always exciting to see startup companies debut their cultured meatballs, sausages and burgers. But I’m looking ahead to what’s next. With a bit more research, time, funding and luck, the cultured meat menu 2.0 will include the steak and pork chops many carnivores know and love.
Natalie R. Rubio, Cellular Agriculture PhD Candidate, Tufts University
Este artículo fue publicado originalmente en The Conversation. Lea el original.

