The deadly, life-giving and transient elements that make up group 15 of the periodic table

The Periodic Table has a dark side and a light side. Chemistry encaptures all what surround us. Knowing this should be of common knowledge.
When you see the periodic table, what comes to mind? The pieces on a scrabble board? Maybe you think about your high school chemistry class. Maybe you think of the colorful table plastered on the wall of a lecture hall in college. Maybe you remember your favorite teacher setting something on fire in the front of the classroom. I am an assistant professor of chemistry at University of Richmond and when I hear the phrase “the periodic table,” I think about life.
I think about how the molecules and chemicals that surround us and dictate our everyday activities are made up of the elements on that table – they sustain our life, they bring beauty to the world and they are vital in medicine.
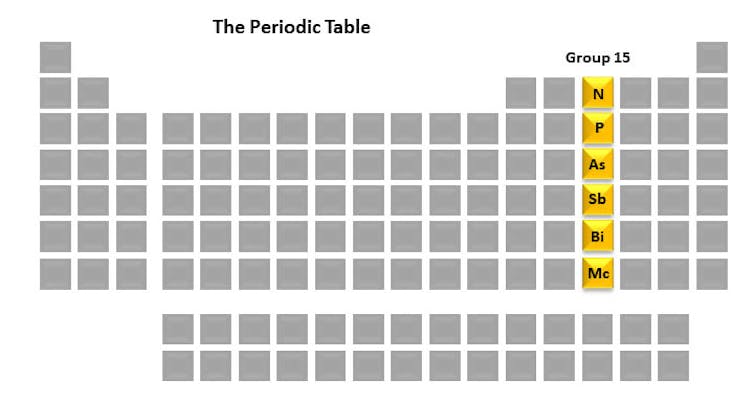 Here is the periodic table with all the elements blocked excepted for the ones in group 15.
Here is the periodic table with all the elements blocked excepted for the ones in group 15.Julie Pollock, CC BY-SA
Each column of the periodic table is called a group. Every member of the group has a similar arrangement of electrons which can result in similar chemical properties. The group 15 elements – nitrogen, phosphorus, arsenic, antimony, bismuth and moscovium – are interesting to me because of their pivotal role in life, as well as in my research lab. One element we study is phosphorus because of its integral role in the fate of cells.
But before we get into those details, let’s take a brief look at each of the group 15 elements. They are a unique set in their history, uses and properties.
Group 15 – giving life and causing death
Nitrogen (N) in its atmospheric form (N₂) makes up approximately 78% of the air we breathe. When bacteria living within plant roots convert it into a usable form through a process called nitrogen fixation, this elemental form of nitrogen gets incorporated into many compounds that are necessary for life – proteins and DNA, for example. At the bottom of the column is Moscovium (Mc), which is interesting because it doesn’t really exist in nature. It’s a radioactive element that can only be generated in a laboratory and survives for less than a second.
Arsenic (As) may be familiar to you because of its association with poisonings. In 1494, Pico della Mirandola, an Italian humanist philosopher during the Renaissance, was poisoned by arsenic, although the details surrounding his early death are still debated. For a long time it was believed that Napoleon Bonaparte died of arsenic exposure in 1821, but after extensive comparisons of preserved hair samples from different stages of his life, researchers concluded the increased levels of arsenic were most likely due to preservation techniques of the time. More recently, the World Health Organization estimated arsenic-contaminated drinking water in Bangladesh resulted in over 9,000 deaths in 2001. How arsenic poisons and kills isn’t completely understood, but there is no doubt that the element causes destruction of vital organs in the human body.
 A woman from Sonargaon, Bangladesh, shows palms affected by years of drinking arsenic-laced water.
A woman from Sonargaon, Bangladesh, shows palms affected by years of drinking arsenic-laced water.AP Photo/ A.M. Ahad
When the element antimony (Sb) is combined with three oxygen atoms to form antimony trioxide, it is used extensively as a flame retardant for furniture, carpets, drapes, rubber, plastics and adhesives. Quantities of this molecule in these household products tend to be very small, and these levels of antimony are regarded as safe.
Bismuth (Bi) is a metal found in the same row of the periodic table as a number of toxic metals; however, compounds containing bismuth are harmless. Bismuth compounds can be found in cosmetics due to their distinctive and desirable silvery shimmer. Even if you haven’t used bismuth-containing personal care products, you have probably encountered it in the well-known antacid Peptobismol®, which is used to treat upset stomachs, or on the Fourth of July when you are watching fireworks. It is a bismuth compound that causes the crackling sounds of the dragon egg fireworks.
Last, but not least, of the group 15 elements is phosphorus (P). It was discovered in 1669 by the alchemist Hennig Brandt and named from the Greek word “phosphoros,” meaning bringer of light. That’s because when the elemental form interacts with atmospheric oxygen it produces a brilliant light. Chemists figured out how to harness the power of this reaction for the development of matches. The red tip on a match still contains a form of phosphorus today.
Phosphates – regulating cancer cell fate
In addition to sparks generated by the element, phosphorus is found in a compound known as a phosphate: phosphorus linked to four oxygen atoms. In cells, when a phosphate molecule is attached to a protein, it can turn on, or activate, the protein so that it can perform its function in the cell – like stimulating growth.
When the phosphate is no longer attached to the protein, the cells stop growing. You can think of it almost like the matches described above – when the phosphate is there, the match can ignite and business can proceed. When the phosphate is removed, the match is just a stick and no light is provided; not as much work can happen in the dark.
In cancerous cells, the phosphate status is out of control. Imagine a lot of lit matches and a very bright room that can result in a flurry of activity. This activity can have severe consequences for cells. For example, unregulated growth and migration can lead to cancer.
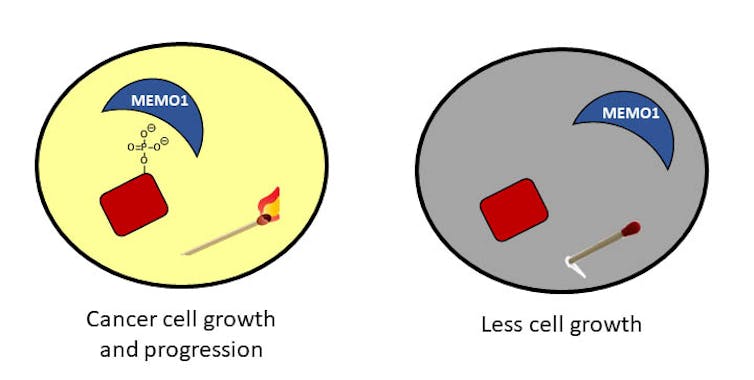 Phosphorylation in cancer cells: When a protein is phosphorylated, it is like a lit match in a room that leads to cancer cell growth. If the phosphate is removed, the match is extinguished and the cells don’t grow as much.
Phosphorylation in cancer cells: When a protein is phosphorylated, it is like a lit match in a room that leads to cancer cell growth. If the phosphate is removed, the match is extinguished and the cells don’t grow as much.In my laboratory at the University of Richmond, we are interested in understanding these phosphates and one protein in particular that interacts with them. This protein, called MEMO1, is found in high quantities in breast cancer patients and helps the phosphates to always stay attached to proteins. We are trying to understand how MEMO1 interacts with these phosphates and are developing strategies to disrupt those interactions.
We hope that our work reveals a way to help remove the phosphates to stop the unchecked growth of cells – in other words, to blow out the matches.
So next time you hear the words “periodic table,” please think of life. Think of the molecules that you encounter every moment of every day, think of the medicine that keeps you healthy and think of those of us who are working to understand how to keep you that way.
Julie Pollock, Assistant Professor of Chemistry, University of Richmond
Este artículo fue publicado originalmente en The Conversation. Lea el original. Photo: Andrew Rafalsky/Shutterstock.com

